Abstract:
The purpose of the investigation is to present an innovating culture medium that can be prepared in basic laboratories. We named the medium PGS and its ingredients are: potato, gelatin and sucralose. Sucralose is an artificial sweetener derived from sucrose that can only be metabolized by small amount of microorganisms. PGS is an innovating medium because it does not require a laboratory for its preparation or use. Moreover, this medium facilitates the growth of fungi at 25°C and delays the growth of bacteria. As an example of its pilot study, we preformed a simple exercise of antimitotic activity of garlic, mint and aloe extracts over Aspergillus niger. In addition, we assessed, qualitatively and quantitatively, sucralose’s possible capacity as an antibacterial agent. The PGS medium was successful for cultivating and growing Aspergillus niger at 25°C for at least two weeks. The results show that only the garlic extract presented an inhibitory effect over the growth of A. niger. Notwithstanding, we observed that sucralose was more successful in delaying the growth and development of Gram-positive bacteria over Gram-negative bacteria. However, more empirical tests are required to determine the extent of the possible antibacterial effect of sucralose in this medium.
Keywords : potato medium, antimitotic activity, sucralose, antibacterial agent
Resumen:
El propósito de esta investigación es presentar un medio de cultivo innovador que se puede preparar en laboratorios básicos. El medio, al cual llamamos PGS, consta de papa, gelatina y sucralosa. La sucralosa es un sustituto dietético del azúcar sacarosa que solo puede ser metabolizado por una cantidad mínima de microorganismos. La novedad del medio PGS es que no requiere de un laboratorio para preparar y trabajar con el medio. Este medio facilita el crecimiento de hongos a 25°C y retrasa el crecimiento de bacterias. Como ejemplo de modelo de estudio, se realizó un ejercicio simple de la actividad antimicótica de extractos de ajo, menta y sábila sobre el crecimiento de Aspergillus niger. Adicionalmente, se evaluó, cualitativamente y cuantitativamente, la posible capacidad de la sucralosa como agente antibacteriano. El medio PGS resultó ser exitoso para el cultivo y crecimiento de Aspergillus niger a 25°C por al menos dos semanas. En adición, se observó que el medio con sucralosa fue más exitoso restringiendo el crecimiento y el desarrollo de bacterias Gram positivas que las Gram negativas. Sin embargo, se necesitan llevar acabo más pruebas empíricas para determinar el alcance del posible efecto antibacteriano de la sucralosa en este medio.
Palabras Claves : medio de papa, actividad antimicótica, sucralosa, agente antibacteriano
Introduction:
Research Problem
Some of the most prevalent limitations of scientific experimentation done in laboratories of Biosafety Level 1 (BSL1) are the difficulty of obtaining materials, the lack of appropriate scientific instruments and the risk of working with dangerous materials. Our proposal presents an experimental model using a new culture medium that permits different essays for evaluating interactions between organisms. This model uses low risk materials commonly found in simple science laboratories and does not require the use of a laboratory above the Biosafety Level 1 (BSL1).
A BSL 1 means the laboratory is not necessarily separated from the general traffic patterns in the building and work is typically conducted on open bench tops using standard microbiological practices. (Richmond & McKinney, 1999) Special containment devices or equipment, such as biosafety cabinets, is not generally required. However, laboratory personnel must have specific training in the procedures conducted in the laboratory and must be supervised by a scientist with training in microbiology or a related science. (1999)
For any microorganism to be used for any purpose, it is necessary to provide the appropriate environment. Culture media are employed in the isolation and maintenance of pure cultures of bacteria and are also used for identification of bacteria according to their biochemical and physiological properties. (Cooper, 2000) However, the manner in which bacteria are cultivated, and the purpose of culture media, varies widely. Liquid media are used for growth of pure batch cultures, while solidified media are used widely for the isolation of pure cultures, for estimating viable bacterial populations, and a variety of other purposes. (2000) Also, a satisfactory microbiological culture medium must contain available sources of carbon, nitrogen, and inorganic salts.
Sucrose is an easily assimilated macronutrient, a substance consumed in high quantities and provides energy. Sucrose is a disaccharide, a union of two monosaccharides or simple sugars, Glucose and Fructose. In humans and other mammals, sucrose is broken down into its constituent monosaccharides by sucrase or isomaltase glycoside hydrolases, which are located in the membrane of the microvilli lining the duodenum. (Schiweck, Clarke & Pollach, 2007; Gray, 1971) However, the accessibility to be metabolized is not found in sucralose.
Sucralose is an artificial sweetener made from the selective chlorination of sucrose (Knight, 1994; Rodero, Rodero & Azoubel, 2009); it changes three molecules of hydroxide for three molecules of Chlorine. A sugar substitute is a food additive that duplicates the effect of sugar in taste, usually with less food energy. (Rodero, Rodero & Azoubel, 2009) Some sugar substitutes are natural and some are synthetic. Those that are not natural are usually called artificial sweeteners. Since it does not cause a caloric increase in mammals, sucralose is considered an artificial sweetener. (1994; 2009)
Due to the human inability to metabolize these molecules, they are passed on to the environment via human excrement. Most artificial sweeteners are found mostly degraded by the wastewater treatment process. (Bauer, et. al., 1999) Sucralose, however, is found in higher concentrations. (Bauer, et. al., 1999; Grice & Goldsmith, 2000) Common mechanical and secondary microbial digestion can only partially mineralize and remove sweetener pollutants. Sucralose’s effect on environmental microbes is largely unknown.
Figure #1: The antimitotic activity of the garlic extract and the fungicide control against Aspergillus niger in the PGS medium. Figure 1 displays the inhibition of Aspergillus niger by the aqueous garlic extract and the clinical fungicide. The garlic extract from tap water represents the extract that was most effective against A. niger. Moreover, Tolnaftate 1% represents the negative control used to compare the results of the extract. There was little inhibition from the control, whereas, the aqueous garlic extract started to hinder mycelial growth at one drop and almost completely inhibited the fungus at 8 drops. The pictures were taken with the Nikon microscope camera system attached to a dissection microscope.
Through in-vitro testing, we propose to demonstrate the resourcefulness of the PGS medium by using vegetable extracts from garlic, mint and aloe against the development of Aspergillus niger. Moreover, we intend to assess the possible antibacterial effect of sucralose over the growth and development of Gram-positive bacteria (Enterococcus faecalis and Staphylococcus aureus) and Gram-negative bacteria ( Pseudomonas aeruginosa, Proteus mirabilis, Enterobacter aerogenes and Escherichia coli) qualitatively and quantitatively, through media inoculation and bacterial spread plates, respectively.
Method:
Materials and Procedure
The Microbiology Laboratory of the University of Puerto Rico, Río Piedras Campus, provided the cultures of A. niger, E. faecalis, S. aureus, P. aeruginosa, P. mirabilis, E. aerogenes and E. coli.
Culture media. The potato gelatin sucralose (PGS) medium is based on 100 mL recipe of a solution containing 12% gelatin, 2 g of sucralose based artificial sweetener and 0.4 g of dehydrated instant potato. Tap water was used as the solvent. The solution was heated and agitated until boil. Three additional culture mediums were used for the evaluation of sucralose. The medium potato gelatin regular sugar (PGRS) is made in the same way as PGS medium except it uses regular sugar (sucrose) instead of the artificial sweetener. The potato dextrose agar (PDA) and trypticase soy agar (TSA) were prepared following the instructions provided by the manufacturer. These are suspend 39 g of prepared medium in 1000 mL of distilled water and 40 g of prepared medium in 1000 mL of distilled water, respectively. The media are then sterilized by autoclaving in 15 PSI at 121°C for 15 minutes.
Plant extracts. The mint and aloe plants were recollected from a humid garden and the garlic was obtained commercially. Different segments of the plants were used to prepare the extracts. The mint extract was made with the leaves. The garlic extract was prepared using peeled garlic cloves. Finally, for the aloe, the leaf segments were peeled to remove the outer crust and only the gelatin substance inside of it was used to make the extract. Once the raw material was assembled, each one was triturated separately in a blender with a solvent in a 1:4 ratio (10 g of organic material in 40 mL of solvent). The solvents were tap water (an aqueous solvent) and ethanol 95% (a non-aqueous solvent). The mixture was blended for 3 minutes. Afterwards, the solution was filtered by gravity through a coffee filter into a 125 mL beaker to remove the solid material. The resulting extract was transferred into a 50 mL sterile tube with screw top and stored in a refrigerator.
Procedure
A gradient method was used with the following doses of the extracts: 0 drops, 1 drop, 2 drops, 4 drops and 8 drops. This step was repeated with each plant extract and the doses were obtained directly from the extracts using disposable sterile pipette. (Ramírez, López, Guzmán & Munguía, 2011) The positive control used was the Petri dish with 0 drops of the extract and the negative control was a clinical antifungal named Tolnaftate 1 % (diluted in a 1:10 ratio with tap water) instead of the plant extracts. Once the Petri dishes were prepared, 1 drop of A. niger was inoculated into the medium with a sterile pipette. The Petri dishes were incubated at 25°C. After an incubation period of 48 hours, the growth diameter of the fungus was measured in centimeters.[1]
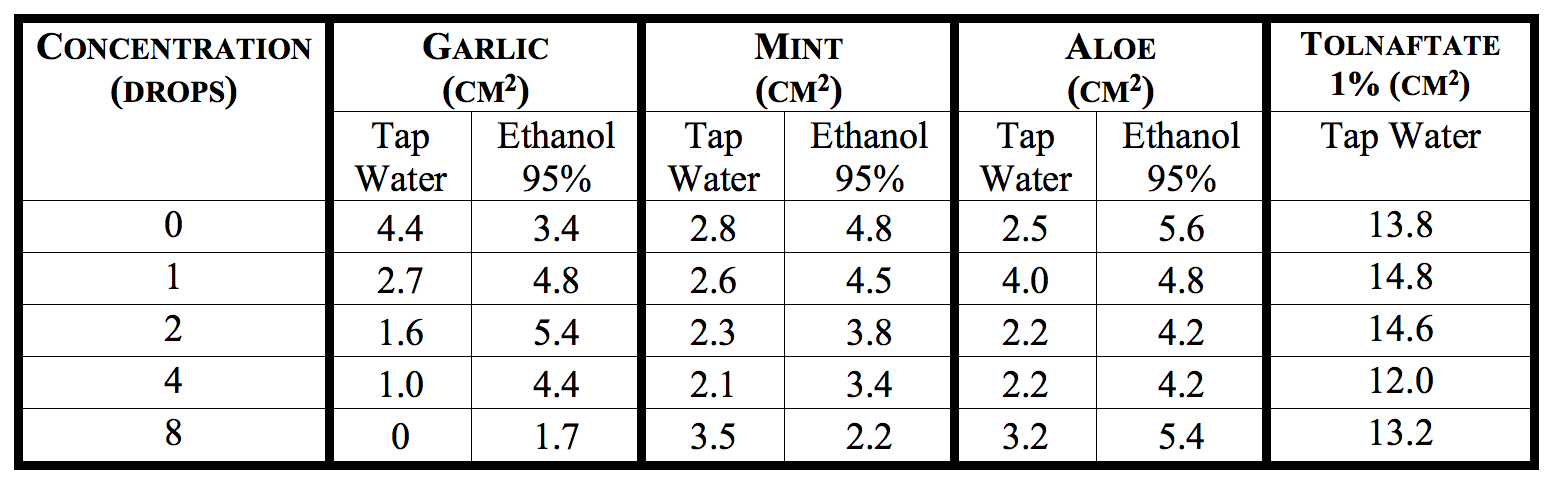
Table #1: Mycelial growth inhibition of Aspergillus niger by the garlic, mint and aloe extracts, and the fungicide control in the PGS medium
Table #1 shows the diameter of the mycelia of Aspergillus niger after being subjected to different antimitotic extracts. The 0 drop corresponds to the positive control and the Tolnaftate 1 % corresponds to the negative control. Moreover, the tap water represents the aqueous solvent and the ethanol 95% represents the non-aqueous solvent. The aqueous garlic extracts shows the most significant inhibition of A. niger, while the negative control, Tolnaftate 1%, barely hinders the growth of the fungus.
Qualitative Method (descriptive)[2] Compartmentalized Petri dishes (Quad plate) were inoculated with the bacteria to determine the difference in growth patterns between the four media. Each plate was inoculated with a single bacterium. Moreover, each of its segments contained one of the four culture media. The media used were Potato dextrose agar as the negative control, trypticase soy agar as the positive control, potato gelatin regular sugar (PGRS), and potato gelatin sucralose (PGS). The culture plates were incubated at 25°C. Moreover, they were examined after 48 hour incubation period with a Nikon microscope camera system attached to a dissection microscope.
Quantitative Method (numeric)[3] Spread plates were made in petri dished from samples of bacteria. This was used to observe the amount of viable colony forming units (CFU) that grew in each of the culture media previously mentioned. The bacteria used to make the spread plates were suspended in a 0.85% saline solution consisting of a 1:100,000 dilution factor. The culture plates were incubated at 25°C. After an incubation period of 48 hours, the colonies were viewed and counted under a Bright field colony counter.
Results
The antimitotic activity of the aqueous and non-aqueous extracts of garlic, aloe and mint over Aspergillus niger in the PGS medium is presented on Table 1 (p. 6). According to Table 1, the non-aqueous plant extracts (ethanol 95%) did not present antimitotic activity. However, the mint extract exhibited some mycelial control over the spreading of A. niger. As shown in Table 1 by a growth reduction of 4.80, 4.50, 3.80, 3.40 and 2.20 cm2 for 0, 1, 2, 4 and 8 drops of the extract, respectively. On the other hand, the only aqueous based extract to significantly inhibit the growth of A. niger was the garlic extract (Figure 1). The results presented in Table 1, show that the growth diameter for the fungus against the extract was of 2.20, 1.10, 0.03, 0 and 0 cm2 for 0, 1, 2, 4 and 8 drops of the extract, respectively. However, the mint and aloe extracts based from tap water did not show a significant inhibitory effect over Aspergillus niger.
The quantitative results found in Figure 2 (p. 8) present the effect that sucralose has over the development and growth patterns of Enterococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis, Enterobacter aerogenes and Escherichia coli in four distinct culture mediums. It also shows that Enterococcus faecalis and Staphylococcus aureus are more inhibited by the PGS medium than both the PDA (potato dextrose agar) and TSA (trypticase soy agar). On the contrary, Pseudomonas aeruginosa , Proteus mirabilis , Enterobacter aerogenes and Escherichia coli grow better in the PGS medium than in the PGRS (potato gelatin and regular sugar) medium (Figure 2). As a rather interesting observation, S. aureus was shown to hydrolyze the gelatin significantly more in the medium with regular sugar than the medium with the artificial sweetener. In addition, P. aeruginosa lost its greenish brown pigmentation in both the PGRS and PGS mediums, which was expressed on the negative and positive controls.
Figure # 2: The qualitative effect of sucralose in the PGS medium against the development and growth patterns of six different species of bacteria. The results refer to the growth patterns of Enterococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, Proteus mirabilis, Enterobacter aerogenes and Escherichia coli in four distinct culture media. They are potato dextrose agar (the negative control), trypticase soy agar (the positive control), potato gelatin and regular sugar, and potato gelatin sucralose. E. coli was the most tolerant to the PGS medium, while, E. faecalis was the least. The pictures were taken with the Nikon microscope camera system attached to a dissection microscope.
In terms of the quantitative results, Table 2 exhibits the number of bacterial colonies that grew on the four media previously stated. It also shows that for the Gram-positive bacteria there were 29 CFU in PGRS compared to the 27 CFU in PGS for Enterococcus faecalis, and16 CFU in PGRS and 21 CFU in PGS for Staphylococcus aureus. On the other hand, Table 2 demonstrates that Pseudomonas aeruginosa grew 8 CFU and 14 CFU, Proteus mirabilis 109 CFU and 124 CFU, Enterobacter aerogenes 110 CFU and 119 CFU, and finally, Escherichia coli 76 CFU and 86 CFU in PGRS and PGS, respectively. It should be noted, however, that the bacterial spreads of P. mirabilis were contaminated with P. aeruginosa.
The qualitative and quantitative tests show that the antibacterial activity of sucralose in the PGS medium has a greater effect on the Gram-positive bacteria than in the Gram-negative bacteria. This is displayed by the inhibition of the development and bacterial growth of colonies in the PGS medium and in the number of colonies that grew in the four different mediums.
Discussion
The simple preparation and easy access are the highlights of the potato gelatin sucralose medium. It is of interest to mention that there was no visible bacterial contamination on the Petri dishes. This is particularly relevant, because the preparation of the medium did not include rigorous aseptic techniques. Moreover, the PGS medium resulted in a successful media for cultivating and growing Aspergillus niger at 25 °C for at least two weeks.
The antimitotic activity of the aqueous and non-aqueous extracts demonstrates that the garlic extract contains an active component that can be effective against A. niger. Moreover, it suggests that the antimitotic component can be obtained through an aqueous solvent. In addition, it should be mentioned that the ethanol 95 % used for the non-aqueous extracts was previously opened. Therefore, there is a possibility that its concentration, and efficiency, have been lowered. Therefore, the results will deviate from their original values. Moreover, the media containing fungicide Tolnaftate 1 % did not show any observable inhibition over the growth Aspergillus niger (Figure 1). This could have occurred because the medium contained samples from a 1:10 dilution already at 1 % concentration. Therefore, the fungicide might have been too diluted to have a concurrent effect on the growth of A. niger. This lowers the application of Tolnaftate 1 % as the negative control for a fungicide against A. niger.
In terms of the qualitative results, the general trend displayed in Figure #2 (p. 8) is that Gram negative bacteria grow better in the potato gelatin sucralose (PGS) medium and that the Gram positive are distinctively more inhibited by said medium. The quantitative results in Table #2 support the trend explained for the qualitative results in Figure 2. That is, the Gram-negative bacteria have a greater facility to grow on the PGS medium than the Gram-positive bacteria. The antibacterial effect of the PGS medium can be attributed to the artificial sweetener, because its components can only be metabolized by a small amount of microorganisms.
Table #2 presents the colony forming units that grew in the four different media. The PDA medium represents the negative control, while, the TSA medium represents the positive control. P. mirabilis, E. aerogenes, and E. coli show greater numbers of CFUs per culture medium in PGS than E. faecalis, S. aureus and P. aeruginosa. However, the spread plates containing Proteus mirabilis were contaminated with Pseudomonas aeruginosa. Therefore, it contains a greater numbers of colony forming units in comparison to the rest of the microorganisms. The bacterial organism used for the bacterial spread plates were taken from a 1:100,000 dilution in a 0.85 % saline solution.
Studies of human oral and gut bacteria have shown an inhibition of bacterial growth in the presence of sucralose (Young & Bowen, 1990). Daily consumption of sucralose can reduce the number and the balance of beneficial bacteria in the gastrointestinal tract by 50 % or more; moreover, bacterial populations do not return to their original level even after a 3-month recovery period (Schiffman & Rother, 2013). They also observed significant reduction in the beneficial bacteria with a dose of 1.1 mg a day per kilogram of body weight (Schiffman & Rother, 2013); this corresponds to the estimated daily intake established by the FDA (FDA, 2006). In principle, a reduction in the beneficial bacteria could cause digestive problems.
Furthermore, if sucralose does inhibit bacterial growth, the type of inhibition would need to be identified as either bactericidal (killing the bacteria) or bacteriostatic (slowing bacterial metabolism), and the mechanisms of such inhibition should be elucidated. (Young & Bowen, 1990; Lange, Scheurer & Brauch, 2012; Sang et al., 2013) The bacteriostatic effect may be due to a decrease in sucrose uptake by bacteria exposed to sucralose. Studies determined that sucralose inhibits invertase and sucrose permease. (Schiffman & Rother, 2013; Lange, Scheurer & Brauch, 2012; Sang et al., 2013) These enzymes cannot catalyze hydrolysis or be effective in transmembrane transport of the sugar substitute.
Finally, our results suggest that this pilot model could be a relatively simple alternative in experiments in laboratories of biosafety level 1 because of its basic preparation and easy accessibility. However, more empirical test should made with a greater number of bacteria to determine the extent of the possible antibacterial effect of sucralose in the PGS medium. Moreover, to identify the type and mechanism the inhibition of sucralose more experimentation is required. As a suggestion for future work, probiotic bacteria should use to evaluate a possible inhibitory effect of sucralose over the beneficial microflora of the intestines.
Notes
[1] The measurements were taken from the fungus mycelium. Moreover, the formula used was: Growth Diameter = (highest diameter ) X (smallest diameter) / 2
[2] In the conventional view by statisticians, qualitative methods produce information only on the particular cases studied, and any more general conclusions are considered propositions. In other words, it deals with descriptions and data that can be observed but not measured.
[3] Quantitative methods are used to seek empirical support for since it deals with numbers and data can be measured.
References cited
Bauer, R., Waldner, G., Fallmann, H., Hager, S., Klare, M., Krutzler, T., Malato, S., & Maletzky, P. The Photo Fenton Reaction and the TiO2 /UV Process for Waste Water Treatment. Catalysis Today. 1999. 53 (1): 131-144. Digital http://www.sciencedirect.com/science/article/pii/S092058619900108X
Cooper, G. Tools of Cell Biology. The Cell: A Molecular Approach. Washington, D.C: ASM Press, 2000. Print
Food and Drug Administration. Food Labeling: Health Claims; Dietary Noncariogenic Carbohydrate Sweeteners and Dental Caries. Federal Register. 2006, 71 (60): 15559-15564. PMID 16572525. Digital http://www.gpo.gov/fdsys/pkg/FR-2007-09-17/pdf/E7-18196.pdf
Gray, M. Intestinal Digestion and Maldigestion of Dietary Carbohydrate. Annual Review of Medicine. 1971, 22: 391-404. Digital http://biblioteca.uprrp.edu:2137/doi/pdf/10.1146/annurev.me.22.020171.002135
Grice, H., & Goldsmith, L. Sucralose, an Overview of the Toxicity Data. Food Chemical Toxicology. 2000, 38: S1-6. PMID 10882813. http://www.sciencedirect.com/science/article/pii/S0278691500000235 http://www.ncbi.nlm.nih.gov/pubmed/10882813
Knight, I. The Development and Applications of Sucralose; a New High-Intensity Sweetener. Canadian Journal of Physiology and Pharmacology . 1994, 72: 435-439. http://biblioteca.uprrp.edu:2078/Search.do?product=UA&SID=1E4R7nFeahQR1VRTBWP&search_mode=GeneralSearch&prID=750f6f5a-3eab-40c4-bf0a-d94edb02b7fe
Lange, F., Scheurer, M., & Brauch, H. (2012). Artificial Sweeteners; a Recently Recognized Class of Emerging Environmental Contaminants: A Review. Anal. Bioanal. Chem. 2012, 403 (9): 2503-2518. Digital http://biblioteca.uprrp.edu:2078/Search.do?product=UA&SID=1E4R7nFeahQR1VRTBWP&search_mode=GeneralSearch&prID=efb82222-50c4-4a5a-bfeb-0a0b7e3e4330
Ramírez, S., López, O., Guzmán, T., & Munguía, S. Actividad antifúngica in vitro de extractos de Origanum vulgare, Tradescantia spathacea y Xingiber officinale sobre Moniliophthora. Tecnología en Marcha. 2011, 24 (2): 3-17. Digital http://dialnet.unirioja.es/descarga/articulo/4835560.pdf.
Richmond, J. Y., & McKinney, R. W. (2009). Biosafety in Microbiological and Biomedical Laboratories. 5th Edition. 4: 30. HHS Publication No. (CDC) 21-1112. Washington, D.C.: U.S. Department of Health and Human Services-Public Health Service Centers for Disease Control and Prevention National Institutes of Health. http://www.cdc.gov/biosafety/publications/bmbl5/BMBL.pdf
Rodero, A., Rodero, L., & Azoubel, R. Toxicity of Sucralose in Humans: A Review. Int. J. Morphol. 2009. 27 (1): 239-244. Digital http://biblioteca.uprrp.edu:2078/full_record.do?product=UA&search_mode=GeneralSearch&qid=3&SID=1E4R7nFeahQR1VRTBWP&page=1&doc=1
Sang, Z. et al. Evaluating the Environmental Impact of Artificial Sweeteners: A Study of their Distribution, Photodegradation and Toxicities.Water Research. 2014, Vol. 52 (April): 260-274. Digital http://www.sciencedirect.com/science/article/pii/S0043135413009019
Schiffman, S., & Rother, K. Sucralose, a synthetic organochloride sweetener: overview of biological issues. Journal of Toxicology and Environmental Health, Part B: Critical Reviews. 2013, 16, 399-451. Digital http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3856475/
Schiweck, H., Clarke, M., & Pollach, G. Sugar. Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim. 2007, Print
Young, D., & Bowen, W. The Influence of Sucralose on Bacterial Metabolism. Journal of Dental Research. 1990, 69 (8): 1480-1484. Digital http://biblioteca.uprrp.edu:2078/full_record.do?product=UA&search_mode=GeneralSearch&qid=6&SID=1E4R7nFeahQR1VRTBWP&page=1&doc=1
Revista [IN]Genios, Volumen 2, Número 1 (septiembre, 2015).
ISSN#: 2324-2747
Universidad de Puerto Rico, Río Piedras
© 2015, Copyright. Todos los derechos están reservados.
![[IN]Genios](http://images.squarespace-cdn.com/content/v1/51c861c1e4b0fb70e38c0a8a/48d2f465-eaf4-4dbc-a7ce-9e75312d5b47/logo+final+%28blanco+y+rojo%29+crop.png?format=1500w)